Abstract
Introduction Cryopreservation of human cellular therapy products (CTPs) is integral for modern blood and marrow transplant (BMT) programs. However, there is limited data available regarding CTP stability during long-term storage, particularly beyond ten years. Our policy assigns a 20-year expiration to cryopreserved HPC(A) and HPC(M) products. To support the 20-year expiration period, a validation program was developed and assessed.
Methods Cryopreserved samples and products stored in vapor phase liquid nitrogen tanks were tested using two sample groups. HPC(A) or HPC(M) specimens from deceased patient samples stored over 15 years were the initial group tested. A second group was added from an ongoing Prospective Stability Program (PSP). Post-thaw viability testing was performed by flow cytometry per our validated procedure based on the ISHAGE gating strategy, producing a percent viability for CD34+ cells (CD34v) and CD3+ cells (CD3v). Acceptance criteria was defined as ≥ 75% viability for both cell populations. Simple linear regression was used to predict the 75% threshold. After initial analysis, data from the PSP was included for an expanded analysis. This provided an additional 14 CD34v and 13 CD3v results, ranging in age from < 1 week (e.g., soon after cryopreservation) to 8 years; all PSP results are from cryovial specimens.
Results Twenty-six collections were identified for testing, collected 15 to 31 years prior. The 25 HPC(A) specimens ranged in age from 15 to 26 years. The single HPC(M) collection was 31 years old (yo). Initially, 11 cryovials, 12 sample bags and 3 product bags from 26 different patients were tested. All but two specimens <20yo were cryovials; one product and one sample bag were also <20yo. All but three specimens >20yo were sample bags; the remainder were products. A second product from the HPC(M) collection was tested four months after the initial test for an additional HPC(M) data point.
All specimens < 20yo and both HPC(M) specimens met the acceptance criteria. Of the 13 HPC(A) over 20yo, nine met the 75% CD34v acceptance criteria (range = 66 - 95%) and four met the 75% CD3v acceptance criteria (range = 42 - 80%). Notably, two HPC(A) only days over 20yo failed to meet CD3v, with results of 52% and 74%; CD34v was 79% and 90%, respectively. However, several 21-22yo met both acceptance criteria. Both HPC(M) results of the 31yo product met acceptance criteria; CD34v mean ± SD = 95% ± 0.4 and CD3v mean ± SD = 86% ± 3.9.
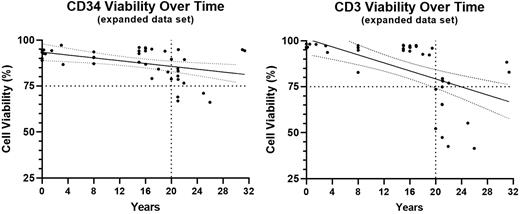
Linear regression analysis of the initial HPC(A) dataset predicts CD34v would reach 75% after more than 30 years and CD3v would reach 75% after 21 years. Similar evaluation of the expanded dataset (which includes stability program samples up to 8 years old as well as both results from the retested HPC(A) and the HPC(M)) predicts reaching 75% CD34v at almost 48 years and CD3v at about 24 years. The accompanying CD34v and CD3v graphs show the expanded dataset and include 95% confidence interval bands for the linear regression analysis.
Conclusions CD34+ cells appear to maintain viability well during cryopreserved storage. CD3+ cell viability is maintained adequately but less robustly compared to CD34+ cells and may begin to decline more dramatically after 20 years of cryopreserved storage. The linear regression analysis supports our current 20-year expiration date. We plan to expand the dataset as the PSP specimens continue to age. We are evaluating viable cell recovery to ensure viability is not artificially high due to cell loss.
Disclosures
Jacob:Amgen: Current equity holder in publicly-traded company; Bristol Myers Squib: Current equity holder in publicly-traded company; Pfizer: Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal